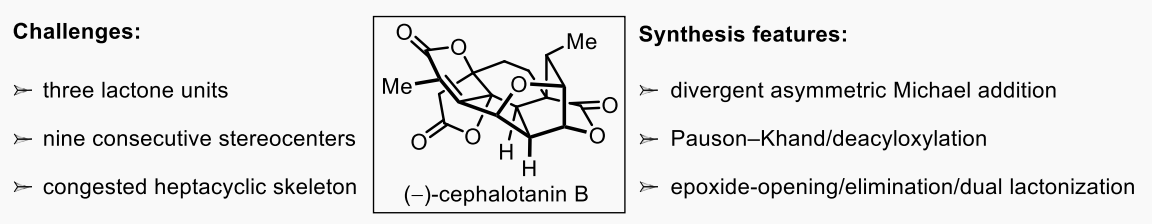
三尖杉萜家族因其突出的生物学特性和独特的分子结构而受到有机合成化学家的广泛关注。近年来,西北大学胡向东课题组基于所发展的桥环张力与缩醛位阻协同调控的立体选择性Pauson–Khand反应策略完成了含有环庚三烯酮结构的(+)-3-deoxyfortalpinoid F、(+)-fortalpinoid A、(+)-cephinoid H (Angew. Chem., Int. Ed. 2021, 60, 18572) 和含有苯环结构的(–)-ceforalide B、(–)-cephanolides B–D (Org. Lett. 2022, 24, 7507) 等多种三尖杉二萜的不对称全合成。近日,胡向东课题组基于一种epoxide-opening/elimination/dual lactonization仿生合成方案,从不对称Michael加成出发,通过新颖的Pauson-Khand/deacyloxylation反应构筑核心四环骨架,再经epoxide-opening/elimination/dual lactonization实现螺旋桨型双内酯结构的构建和拥有异常拥挤七环结构和九个连续立体中心的(–)-cephalotanin B的对映选择性全合成(Angew. Chem. Int. Ed. 2023, e202312599)。
Enantioselective Total Synthesis of (–)-Cephalotanin B
Zezhong Sun,† Shuang Jin,† Jianing Song, Lihua Niu, Fan Zhang, Han Gong, Xin Shu, Yunxia Wang, Xiangdong Hu*Angew. Chem. Int. Ed. 2023, e202312599.
